T1D Clinic
Ulna
Development Platform for Artificial Pancreas Algorithms
-
About &
Objectives -
Patient
Modeling & Data -
Experiment
& Study Design -
Integrating control
algorithms -
Results
& Reports
About & Objectives
- Clinical trials are an integral part of the development process of the artificial pancreas (AP).
- Preclinical testing with computer simulations facilitates and accelerates this process.
- Our powerful simulation system mimics the glucose responses of T1D patients under various physiological conditions to enable testing and validating single and dual-hormone AP control algorithms.
Patient Modeling & Data
- The development platform utilizes a novel glucoregulatory system of ODEs to mimic patient responses.
- System currently includes 15 T1D patients. Each virtual patient is connected to a real patient that was tested in a clinical trial.
- Patients' parameters were generated by cloning techniques using WinBUGS. Our model takes into account inter and intra patient variability of glycemic responses.
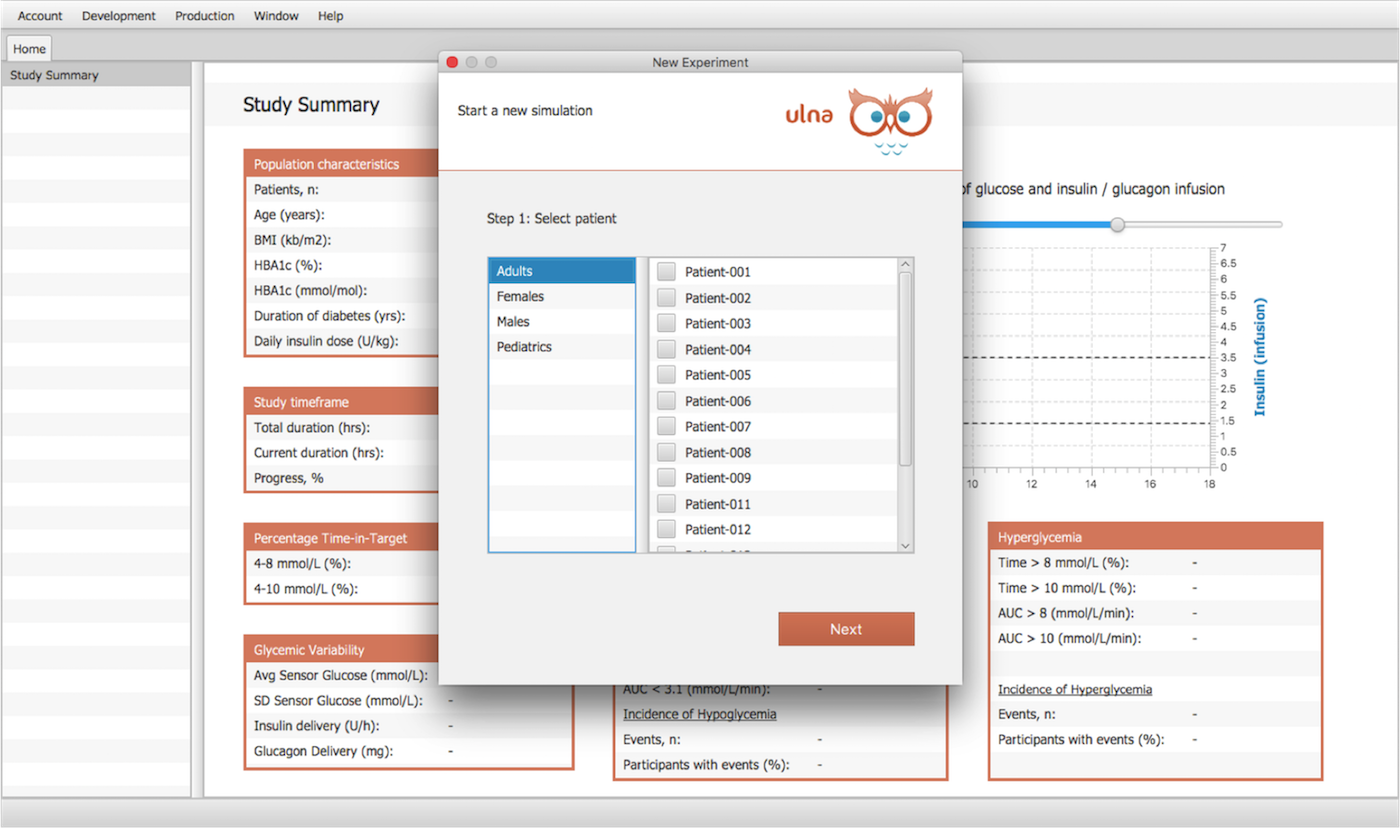
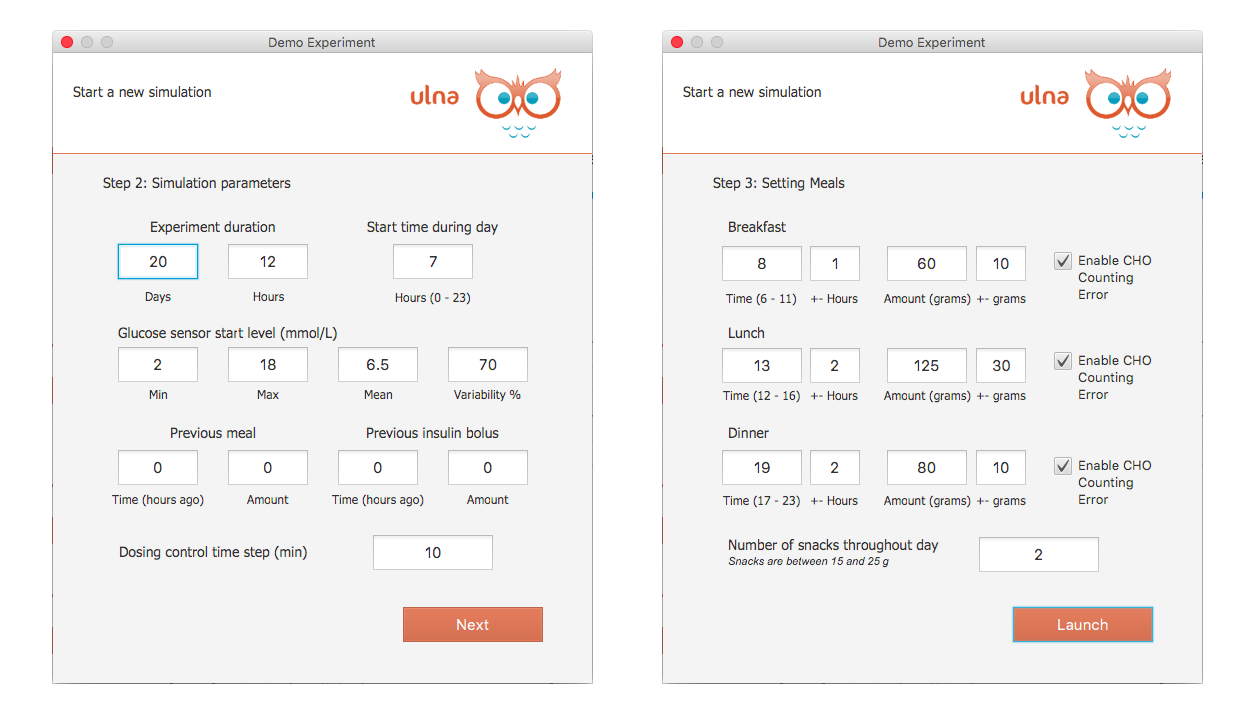
Design your studies with these parameters:
- Number of Patients
- Study duration (days)
- Start time during day
- Variability of glucose start level
- Previous meals and boluses
- Dosing time step
- Breakfast, lunch, dinner and snack time and amount variability during study
- Option of CHO counting error
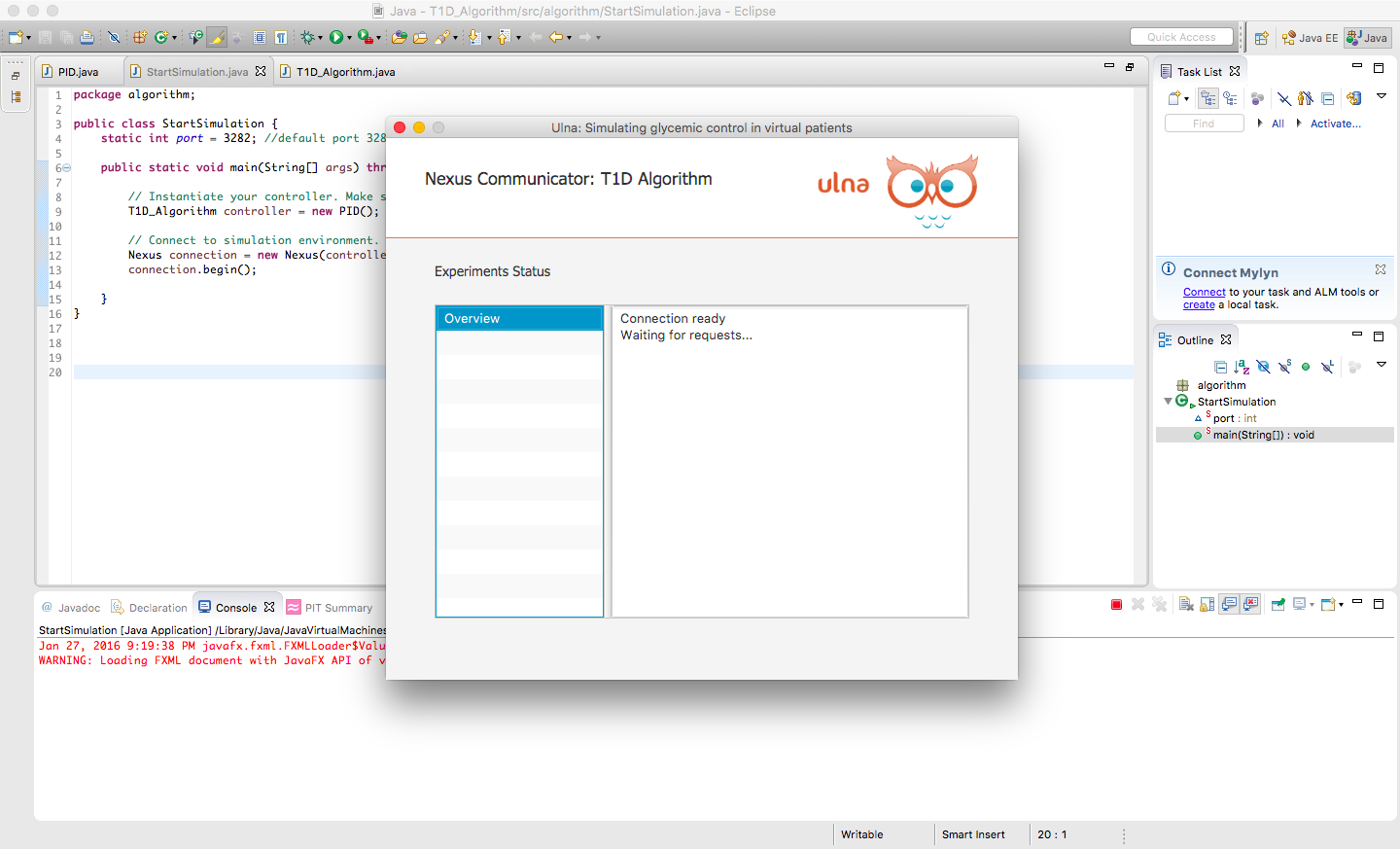
Your Control Algorithm
- Platform supports control algorithms for single and dual-hormone systems.
- User algorithms are completely decoupled from the system to allow for code separation and security.
- Control algorithms run independently from the platform and communicate through Nexus, our client / server communication handler protocol.
- Algorithm calculations are performed on your local machine, while patient responses to dosing results of your control algorithms are calculated in the cloud.
- Users can assess and modify various dosing control strategies without having to ship or load code into the platform.
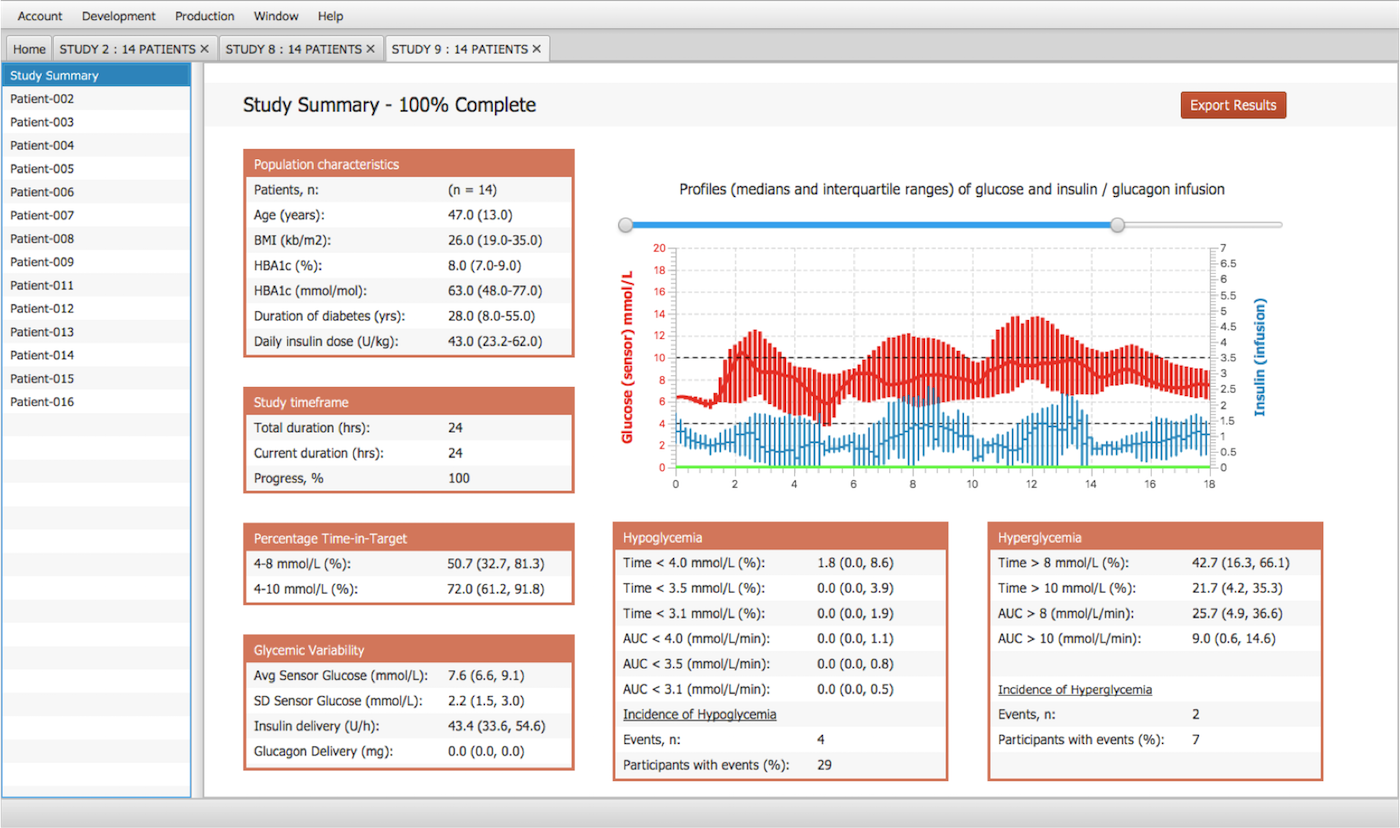
Results & Reports
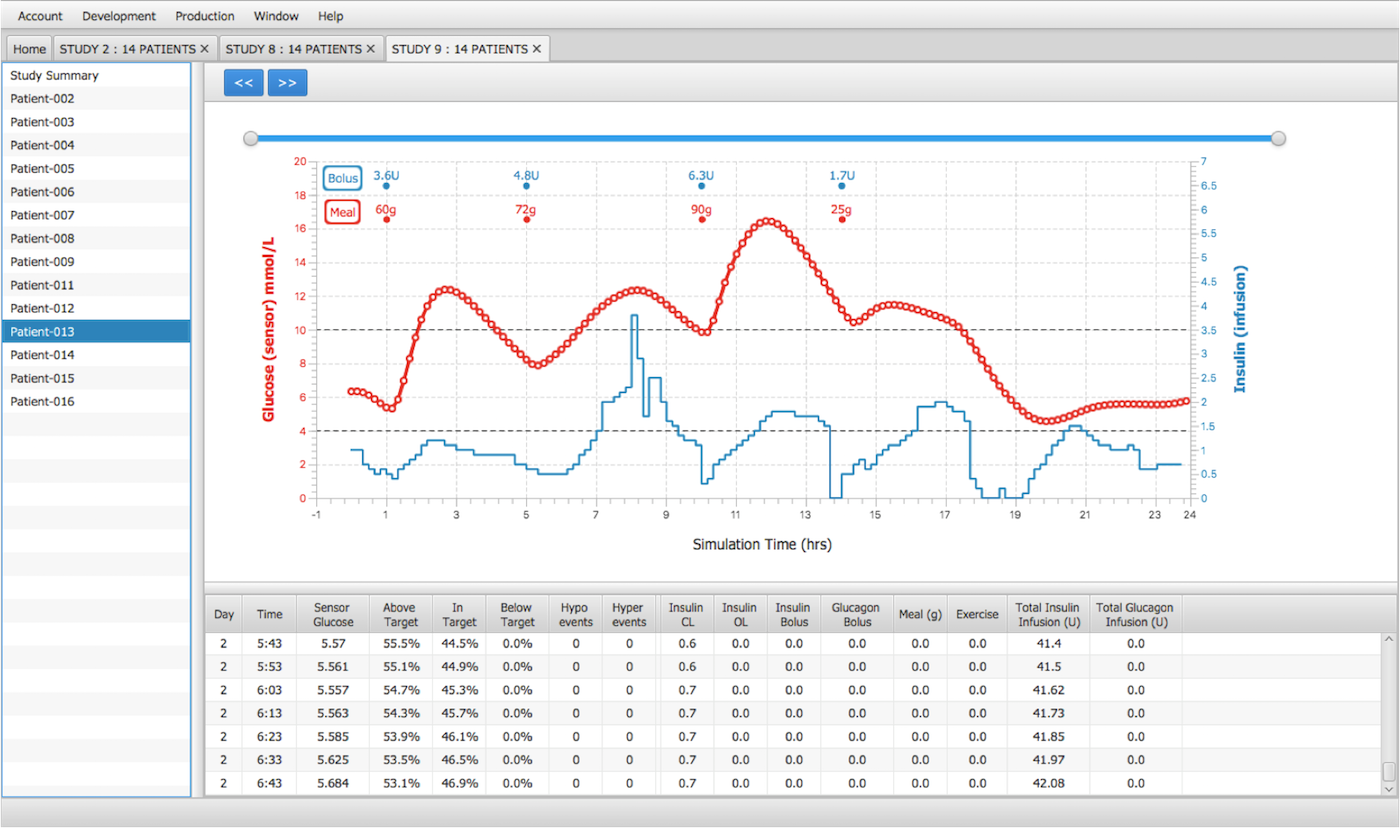
- The development platform is built on object-oriented designs and runs batch simulations in a concurrent multi-threaded environment.
- In addition to overall study outcomes, users can observe the detailed results of every patient.
- The following outcomes are compiled in a report (on screen & MS Excel): Population Characteristics, Time in target, Glycemic variability, Hypoglycemia & Hyperglycemia summaries.
Featured beta users